Abstract
Introduction: B-acute lymphoblastic leukemia (B-ALL) patients that harbor rearrangements of the Mixed-lineage leukemia gene ( MLLr ) have a particularly dismal clinical outcome. Although it is known that this oncoprotein radically alters epigenetic regulation, the precise molecular mechanisms that define the cellular phenotype of these cancers remain poorly understood and new therapeutic strategies are sorely needed. Furthermore, although flow cytometric immunophenotyping has been performed for decades on B-ALL blasts, relatively little is known about the cell surface proteome of these cancers outside of canonical CD markers. Due to its role in modulating cellular behavior as well as accessibility to immunotherapy, the cell surface represents an attractive target for unbiased proteomic profiling in order to gain insight into cellular behavior as well as enabling the development of targeted therapies. Here, we aim to resolve the cell surface proteomic landscapes of MLLr and non- MLLr B-ALL cellular models. Our goal is to generate new mechanistic insights into the molecular re-wiring that occurs in these forms of cancer and identify novel candidates for targeted immunotherapy.
Methods: We examined 10 B-ALL cell lines that harbor different oncogenic fusions including the MLL-AF4, BCR-ABL, and ETV6-RUNX1 translocations, respectively, using both "classical" lines as well as low-passage patient-derived lines, in addition to non-malignant B-cells as a control. We performed unbiased labeling of cell surface proteins by biotinylation of glycosylated amino acids (3 biological replicates each) followed by affinity pull-down with streptavidin. After on-bead trypsin digestion, peptides from bound glycoproteins were analyzed on a Thermo Q-Exactive Plus Mass spectrometer using 4-hour LC runs, data-dependent acquisition, and label-free quantification to identify and quantify members of the cell surfaceome for each cell line. To confirm our proteomic findings we performed flow cytometry using a BD CytoFLEX flow cytometer on select cell lines with fluorescently conjugated monoclonal antibodies, in order to assess presence and qualitative abundance of particular cell surface targets.
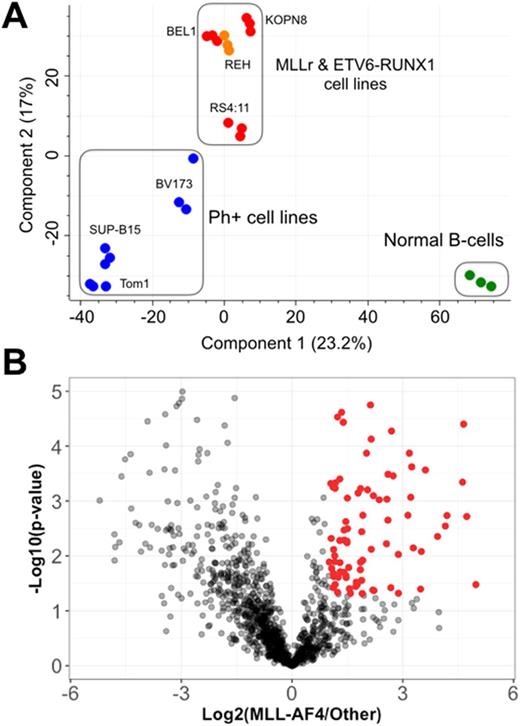
Results: We quantified on average >900 high-confidence membrane proteins (FDR=0.05) per cell type, comprising ~40% of all proteins identified in each experiments. Principal component analysis identified unique cell surfaceome signatures that distinguish several of the leukemia subtypes and normal B-cells from each other, implying different cell-surface phenotypes associated with specific B-ALL genetic alterations (Figure 1A). As positive controls, we found several proteins differentially expressed in MLLr that were also previously identified based on transcriptomic profiling of MLLr patient samples, such as FLT3 and PROM1. In our data, the MLLr surfaceome was surprisingly enriched for Ephrin receptors, matrix metalloproteases, and other proteins that play a role in cell motility, invasion of tissues, and modulation of the tumor microenvironment (significantly enriched membrane proteins colored in red, Figure 1B). Using flow cytometry with monoclonal antibodies specific for select cell surface markers we found excellent agreement with our proteomic data. We will next move to patient-derived xenografts and tissues for further validation. These findings suggest these novel markers may be candidates for downstream immunotherapy development, potentially specific to MLLr B-ALL, where there is particularly high clinical need.
Summary and Conclusions: In this work, we profiled the cell surface proteomes of multiple B-ALL cell models, identifying cell surfaceome signatures unique to different B-ALL subtypes. Overall, our work demonstrates the power of performing un-biased interrogation of the tumor surfaceome to identify new therapeutic targets for immunotherapy, as well as provide a resource to advance our phenotypic understanding of these aggressive cancer subtypes.
Wiita: TeneoBio, Inc.: Research Funding; Sutro Biopharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal